标题 | 发表场所 | 类型 | 时间 |
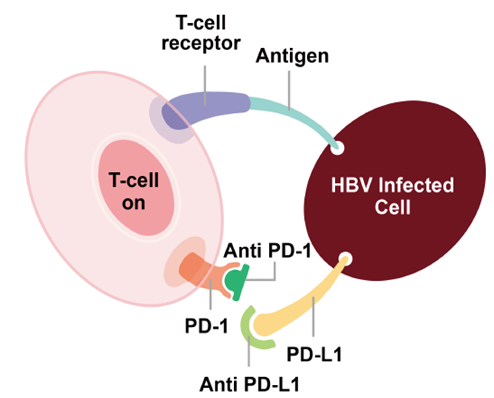
HBsAg Loss in Chronic Hepatitis B Patients After 24-Week Treatment with Subcutaneously Administered PD-L1 Antibody ASC22 (Envafolimab): Interim Results from a Phase IIb Extension Cohort | 2023年美国肝病研究协会(AASLD)年会(The Liver Meeting® 2023) | 海报 | 11/2023 |
| Anti-PD-L1 antibody ASC22 in combination with chidamide potentiates HIV latency reversal and immune function from ART-suppressed individuals: a single center,single-arm,phase Ⅱ study | 第12届IAS艾滋病毒科学会议(The 12th International AIDS Society (IAS) Conference) | 海报 | 7/2023 |
| A phase 2 study of ASC42, a novel farnesoid X receptor (FXR) agonist, in combination with PEGylated interferon (PEG-IFN) and entecavir (ETV) in chronic hepatitis B patients with 12-week treatment | 欧洲肝脏研究协会(EASL)2023年国际肝脏大会(The International Liver Congress™ 2023) | 海报
| 6/2023 |
In vivo evaluation of ASC10 against SARS-CoV-2 virus in K18-hACE2 mouse SARS-CoV-2 model | 2023年第33届欧洲临床微生物与传染病学会年会(The 33rd ECCMID 2023) | 海报
| 4/2023
|
| Effects of Subcutaneous PD-L1 Antibody ASC22 (Envafolimab) Plus nucleos(t)ide analogs on HBsAg reduction in patients with chronic hepatitis B infection are correlated with pre-treatment HBsAg level | 2023年亚太肝脏研究协会(APASL)年会(APASL Annual Meeting 2023) | 口头报告 | 2/2023 |
ALT flares were linked to HBsAg reduction, seroclearance and seroconversion: interim results from a Phase IIb study in chronic hepatitis B patients with 24-week treatment of subcutaneous PD-L1 antibody ASC22 (Envafolimab) plus nucleos(t)ide analogs | 欧洲肝脏研究协会(EASL)2022年国际肝脏大会(The International Liver Congress™ 2022) | 口头报告 | 6/2022 |
| HBsAg Loss in Chronic Hepatitis B Patients with Subcutaneous PD-L1 Antibody ASC22 ( Envafolimab ) plus Nucleos (t)ide Analogs Treatment : Interim Results from a Phase IIb Clinical Trial | 2021年美国肝病研究协会(AASLD)年会(The Liver Meeting® 2021) | 口头报告 | 11/2021 |
| A Phase IIa trial of Subcutaneously Administered PD-L1 Antibody ASC22 (Envafolimab) in Patients with Chronic Hepatitis B | 2021年美国肝病研究协会(AASLD)年会(The Liver Meeting® 2021) | 口头报告 | 11/2021 |
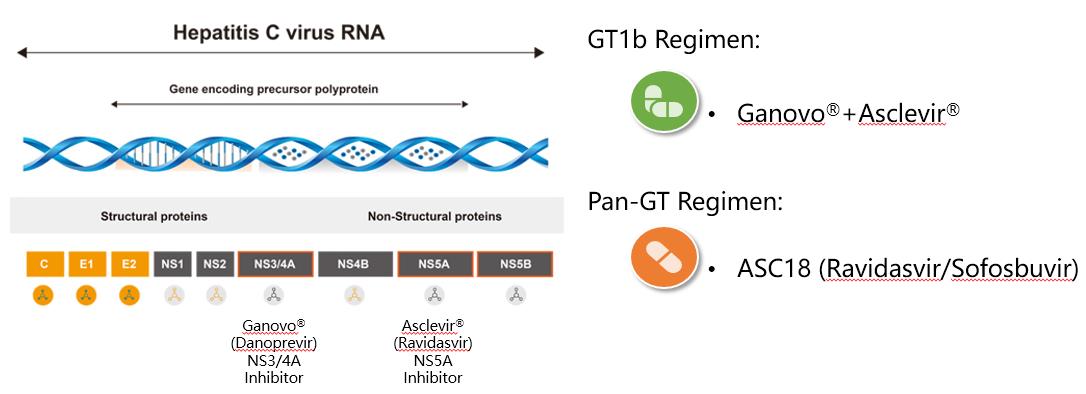
| Efficacy and safety of ravidasvir plus sofosbuvir in patients with chronic hepatitis C infection without cirrhosis or with compensated cirrhosis (STORM-C-1): interim analysis of a two-stage, open-label, multicentre, single arm, phase 2/3 trial | The Lancet Gastroenterology & Hepatology, Volume 6, Issue 6. | 论文 | 04/2021 |
Significant in vitro and in vivo inhibition of HBsAg and HBV pgRNA with ASC42, a novel non-steroidal FXR agonist | 欧洲肝病学会(EASL)年会 (International Liver Congress™ 2021) | 海报 | 04/2021 |
| First clinical study using HCV protease inhibitor danoprevir to treat COVID-19 patients | Medicine (2020) 99:48 | 论文 | 11/2020 |
| 利托那韦强化的达诺瑞韦联合达拉他韦治疗1b型慢性丙型肝炎的临床疗效分析 | 中华临床感染病杂志, 2019(05):350-351-352-353-350-353,371. | 论文 | 10/2019 |
| Efficacy and Safety of All-oral, 12-week Ravidasvir Plus Ritonavir-boosted Danoprevir and Ribavirin in Treatment-naïve Noncirrhotic HCV Genotype 1 Patients: Results from a Phase 2/3 Clinical Trial in China | Journal of Clinical and Translational Hepatology 2019;7(3):213-220 | 论文 | 09/2019 |
| Efficacy and safety of 12-week interferon-based danoprevir regimen in patients with genotype 1 chronic hepatitis C | Journal of Clinical and Translational Hepatology 2019;7(3):221-225 | 论文 | 07/2019 |
| All-oral, 12-week ravidasvir plus ritonavir-boosted danoprevir and ribavirin delivers 100% svr12 in treatment-naive noncirrhotic hcv genotype 1 patients with resistance-associated substitutions of a phase 2/3 clinical trial in China | Journal of Hepatology 2019 vol. 70 | e141–e382 | 海报 | 04/2019 |
| 12 week Ravidasvir plus ritonavir-boosted Danoprevir and ribavirin achieves 99% SVR12 in treatment-naı ¨ve non- cirrhotic HCV GT1 patients: Subanalysis of phase 2/3 clinical trial in China | Hepatol Int (2019) 13 (Suppl 1):S86 | 摘要 | 02/2019 |
| 达诺瑞韦及其联合PR方案治疗慢性丙型肝炎研究进展 | 中华临床感染病杂志,2018,11(02):84-89. | 论文 | 04/2018 |
| Twelve-week Ravidasvir plus ritonavir-boosted Danoprevir and ribavirin for non- cirrhotic HCV genotype 1 patients: A phase 2 study | Journal of Gastroenterology & Hepatology, 2018, 33(8). | 论文 | 01/2018 |
| The Effcacy and Safety of All-Oral, 12-Week Ravidasvir Plus Ritonavir-Boosted Danoprevir and Ribavirin in Treatment-Naïve Non-Cirrhotic HCV Genotype 1 Patients : Results from a Phase 2/3 Clinical Trial in China | 2018年美国肝病研究协会(AASLD)年会(The Liver Meeting® 2018) | 海报 | 11/2018 |
| MAKALU: Twelve-week of treatment with Ritonavir-boosted Danoprevir Pluzs Peginterferon and Ribavirin produces 96% SVR12 in HCV Genotype 1-Infected Non-Cirrhotic Chinese Patients | Hepatol Int (2017) 11 (Suppl 1):S190 | 海报 | 02/2017 |
| Ritonavir-boosted danoprevir plus peginterferon alfa-2a and ribavirin in Asian chronic hepatitis C patients with or without cirrhosis | Journal of Gastroenterology and Hepatology 31 (2016) 1757–1765 | 论文 | 10/2016 |
| DAUPHINE: a randomized phase II study of danoprevir/ritonavir plus peginterferon alpha-2a/ribavirin in HCV genotypes 1 or 4 | Liver International,2015,35(1):108-119. | 论文 | 01/2015 |