新力莱®
2020年7月29日,由歌礼开发的全口服丙肝治疗方案获得中国国家药品监督管理局批准上市。公司的全口服丙肝治疗方案(RDV/DNV 治疗方案)是由拉维达韦(新力莱®)联合达诺瑞韦(戈诺卫®)组成。
拉维达韦是歌礼第二个已上市的慢性丙肝药物,是一种同类最佳的,针对丙肝NS5A靶点的泛基因型DAA。已经完成II/III期临床研究。RDV/DNV治疗方案(拉维达韦联合达诺瑞韦及利巴韦林),是一种全口服无干扰素的慢性丙肝治疗方案,治愈率 (SVR12) 达99%。相比含聚乙二醇干扰素和利巴韦林治疗方案安全性更高,且持续治疗时间短,仅为12周。RDV/DNV治疗方案相比起目前已被批准的Daklinza/Sunvepra方案显示出更高的耐药屏障,就基线NS5A耐药突变的患者而言,II/III期临床试验已表明,RDV/DNV治疗方案的治愈率(SVR12)达100%。
新力莱®的优势
根据临床试验结果,RDV/DNV治疗方案有可能在以下方面解决当前HCV主要治疗方案面临的局限性:
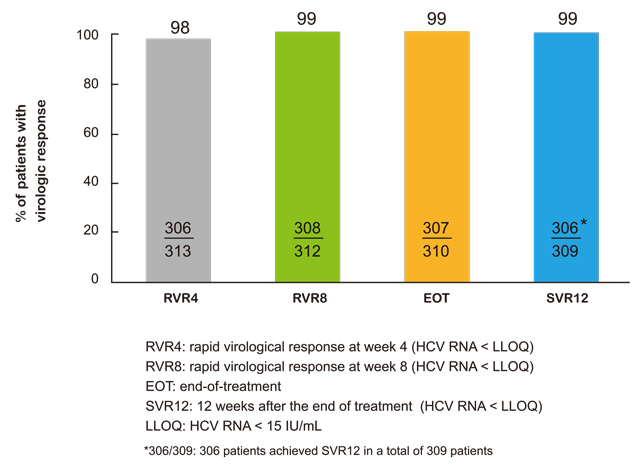
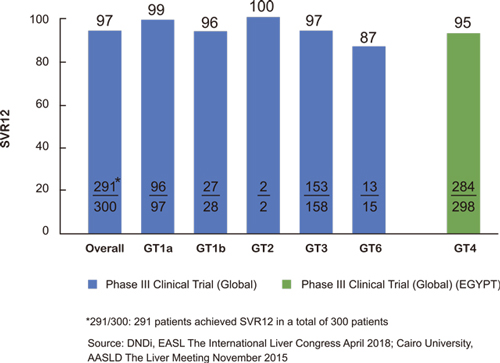
• 同类最佳NS5A抑制剂。在中国进行的II/III期临床试验中有309位HCV基因1型患者,RDV/DNV治疗方案的治愈率(SVR12)达 99%。下图为该临床试验不同阶段的疗效(RVR4、RVR8、EOT及 SVR12)。
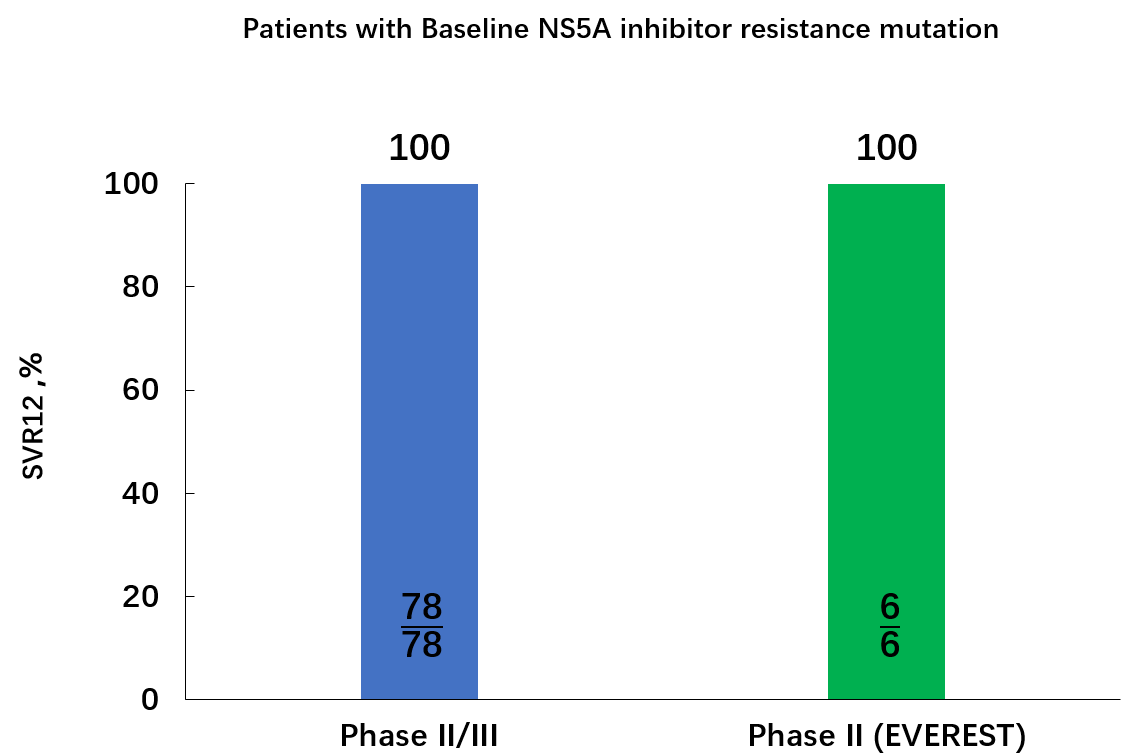
• 对基线NS5A耐药替换(RASs)的CHC患者非常有效。 RDV/DNV治疗方案对II/III期临床试验中存在基线 NS5A RASs 的CHC患者治愈率(SVR12)达100%。在II期临床试验 (EVEREST研究)中有六名患者发生基线NS5A RASs,6名患者全部获得SVR12。在中国,约有19%的丙肝患者存在基线 NS5A RASs。竞争对手产品在治疗受基因1b型HCV感染且含基线 NS5A耐药突变的患者时显示的治愈率只有20% (SVR12)。下图为RDV/DNV治疗方案II/III期临床试验及 II期临床试验 (EVEREST)中基线NS5A RASs的患者的SVR12。
• 对难治的基因型有效。在国外进行的RDV/SOF治疗方案II/III期临床试验表明,基因1a型患者的治愈率(SVR12)达99%,而基因3型患者的治愈率(SVR12)达97%。下图为RDV/SOF方案II/III期临床试验中难治基因型HCV感染患者的SVR12。
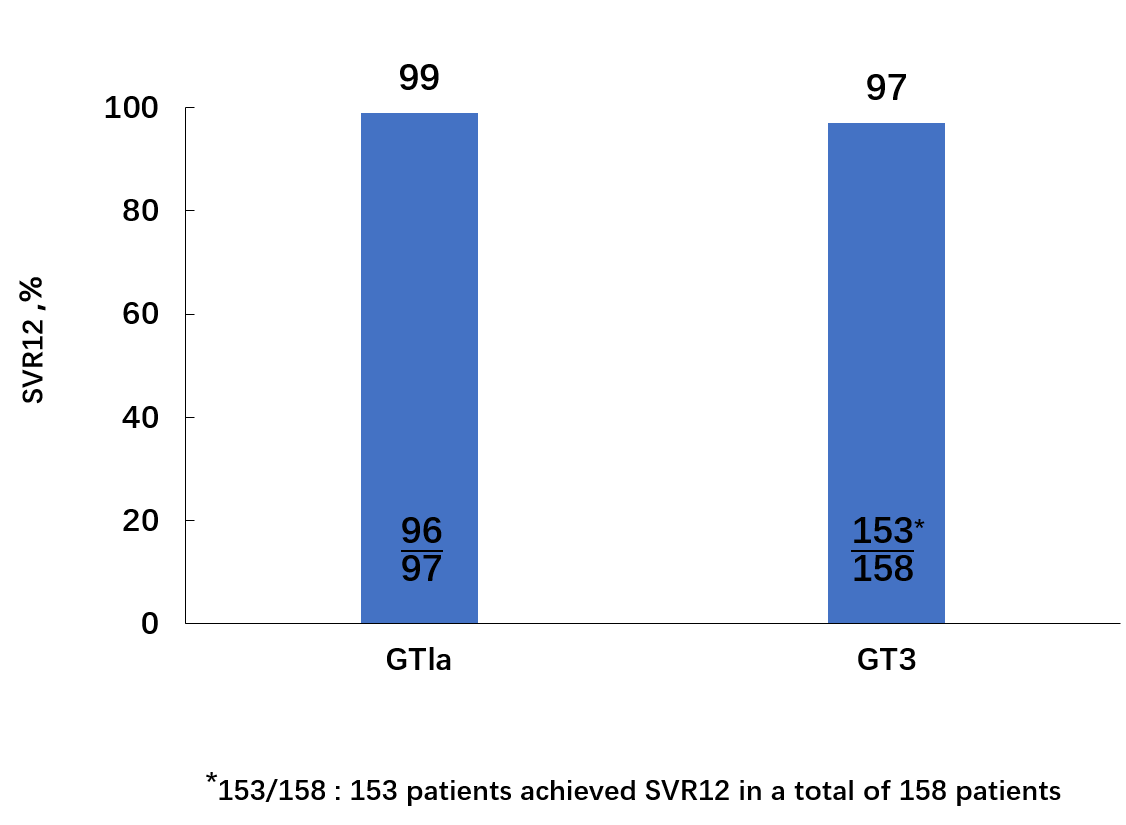
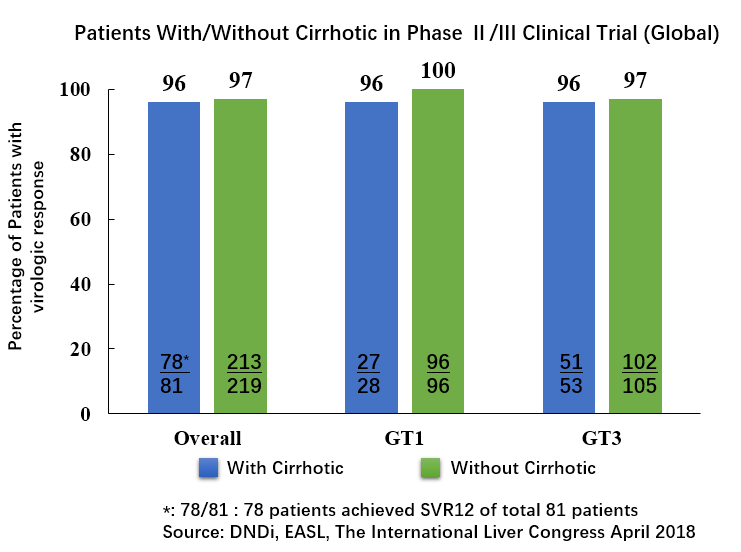
• 对合并肝硬化患者有效。在国外进行的RDV/SOF治疗方案II/III期临床试验表明,对合并肝硬化的基因1型及基因3型HCV感染患者的治愈率(SVR12)达96%。下图为RDV/SOF方案II/III期临床试验中合并肝硬化患者的SVR12。
• 对 HCV/HIV合并感染患者有效。在国外进行的RDV/SOF治疗方案II/III期临床试验表明,HCV/HIV合并感染患者的治愈率 (SVR12)达97%。下图为RDV/SOF方案II/III期临床试验中HCV/HIV双重感染患者的SVR12。

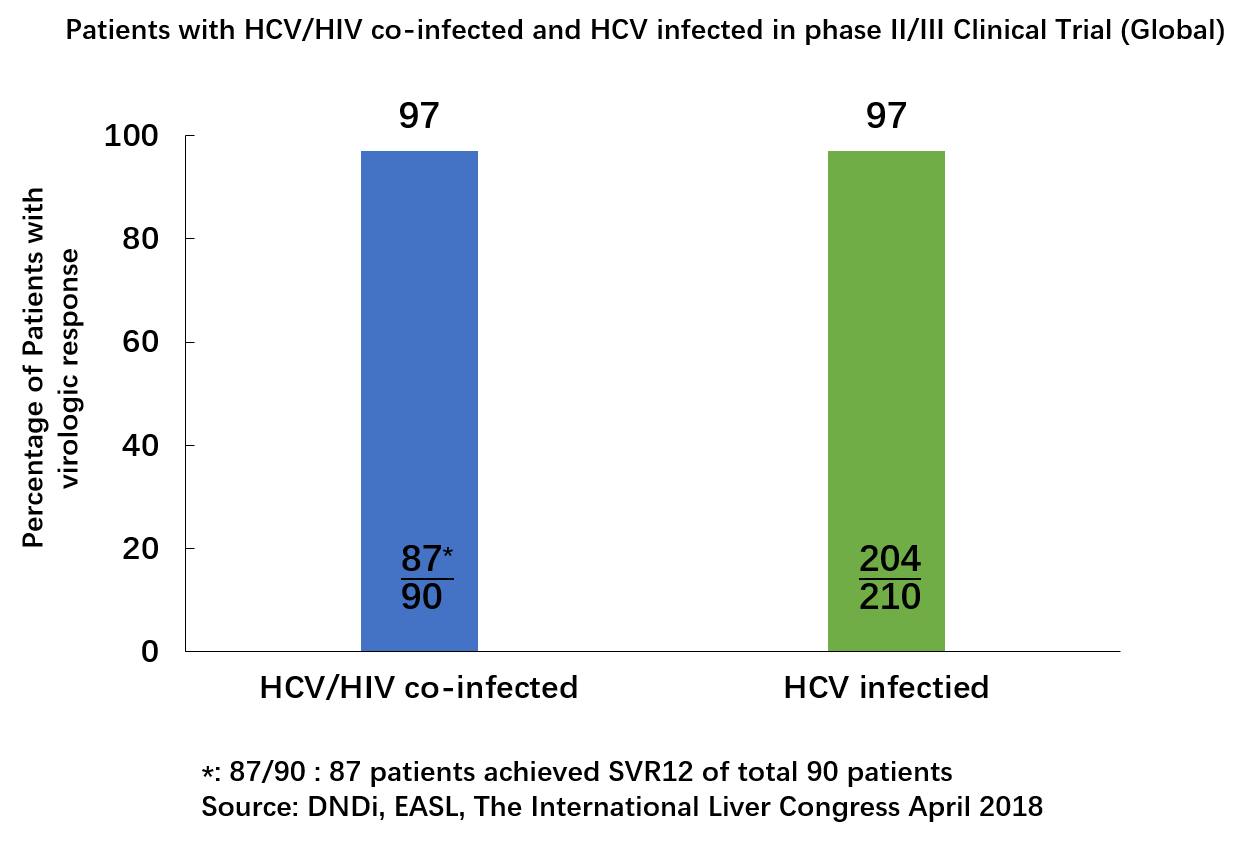
• 对1至6型基因具有泛基因型抗病毒活性。体外研究表明,拉维达韦对 HCV基因1至6型具有有效抗病毒活性。在国外进行的RDV/SOF治疗方案的两项II/III期临床试验均表明,基因1、2、3及6型的总体治愈率(SVR12)达97%,而基因4型的治愈率 (SVR12)达95%。下图为RDV/SOF方案II/III期临床试验中以下所示HCV基因型的SVR12。
• 卓越的安全性及耐受性。在中国进行的II/III期临床试验已表明,RDV/DNV治疗方案安全且耐受性较好,并未出现治疗相关的严重不良反应。除贫血及高尿酸血症外,RDV/DNV治疗组与安慰剂对照组出现的不良反应相似。RDV/DNV治疗组贫血发生率较高可能与使用利巴韦林相关。